Background information
The IPA/Erasmus MC Pompe Survey (’the Pompe Survey’) collects information on the effects of Pompe disease on patients’ lives, and how these may change with treatment. Patients themselves provide this information through an annual questionnaire. Launched in 2002, the Pompe Survey is a collaboration of the International Pompe Association (IPA) and Erasmus MC. Over the past two decades, the Pompe Survey has given us new insights, allowing us for example to quantify the rate of disease progression and the positive effects of enzyme replacement therapy (ERT) on patients’ quality of life and survival.
Why do we need the Pompe Survey?
The information collected in the Pompe Survey gives us better insight into what happens to patients over time. How does the disease progress and how does treatment affect this? Each year, patients report the physical problems they experience, their quality of life and the treatment they receive.
This information is key to studying the effects of treatment on patients’ lives. This remains relevant as new treatments are being approved for use. In addition, the information helps to inform physicians about the (changing) needs of patients.
In the future we hope to combine these self-reported outcomes with clinical measurements of these patients. Combined, these data provide the full picture needed to evaluate treatment strategies.
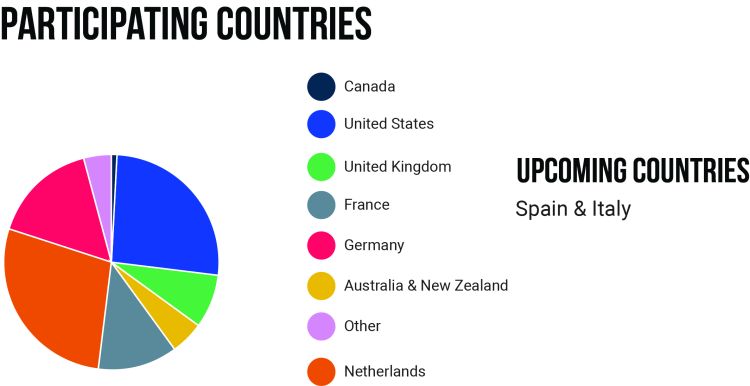
Participation worldwide
In total, 538 patients ever participated in the Pompe Survey. At this moment we have patients participating from Australia, Belgium, Canada, France, Germany, the Netherlands, New Zealand, Switzerland, the United Kingdom and the United States.